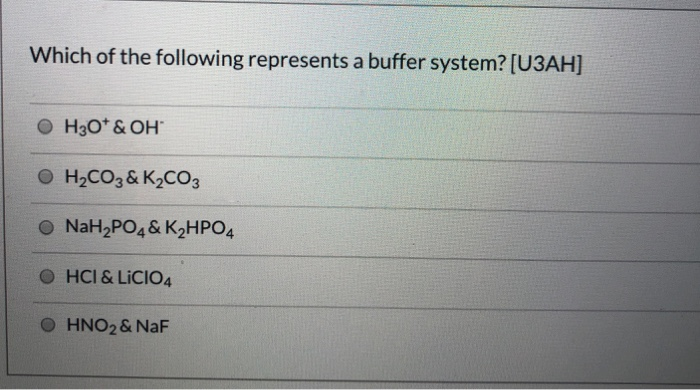
Which Of The Following Represents A Buffer System?
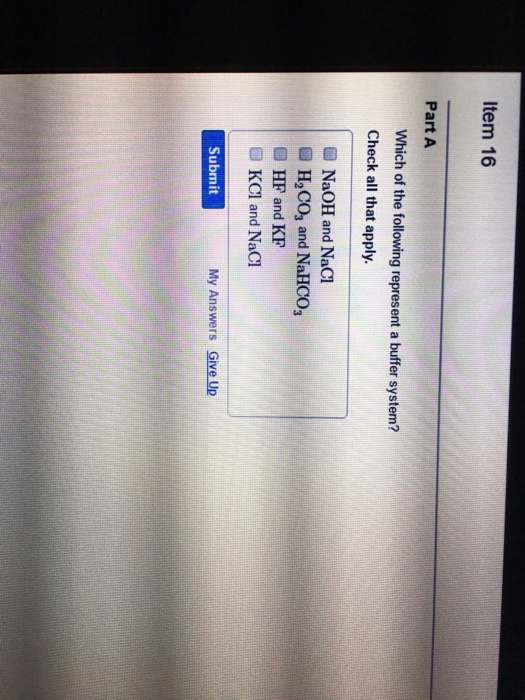
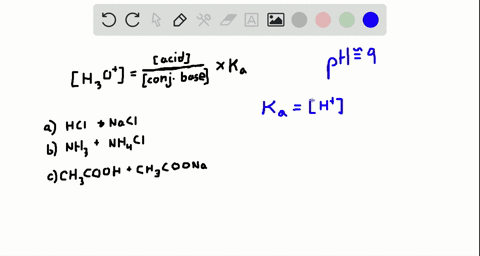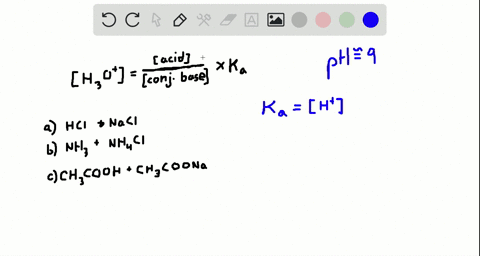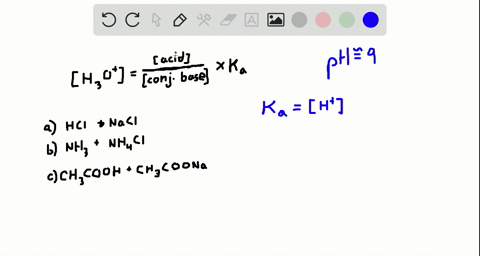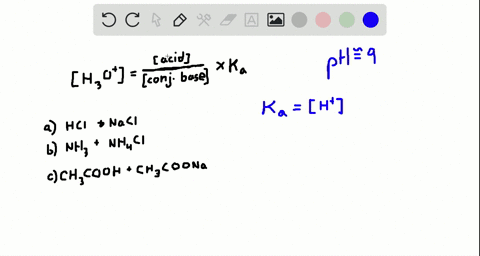
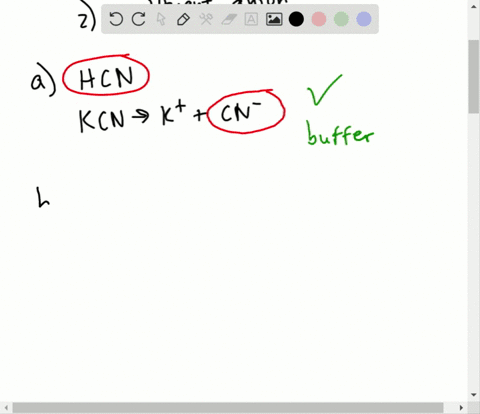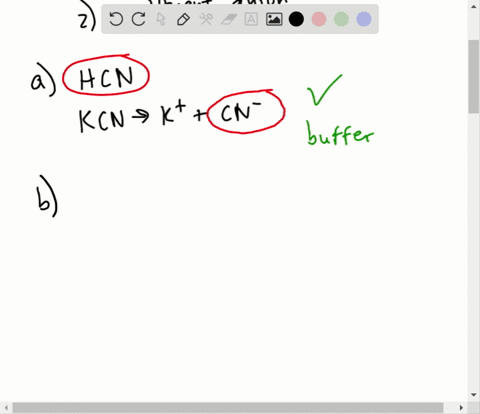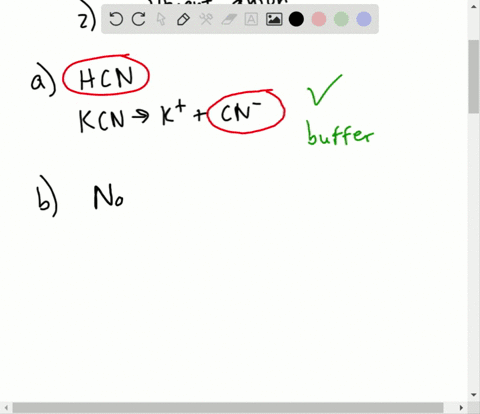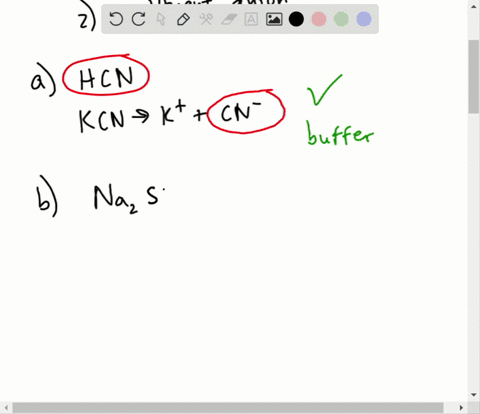
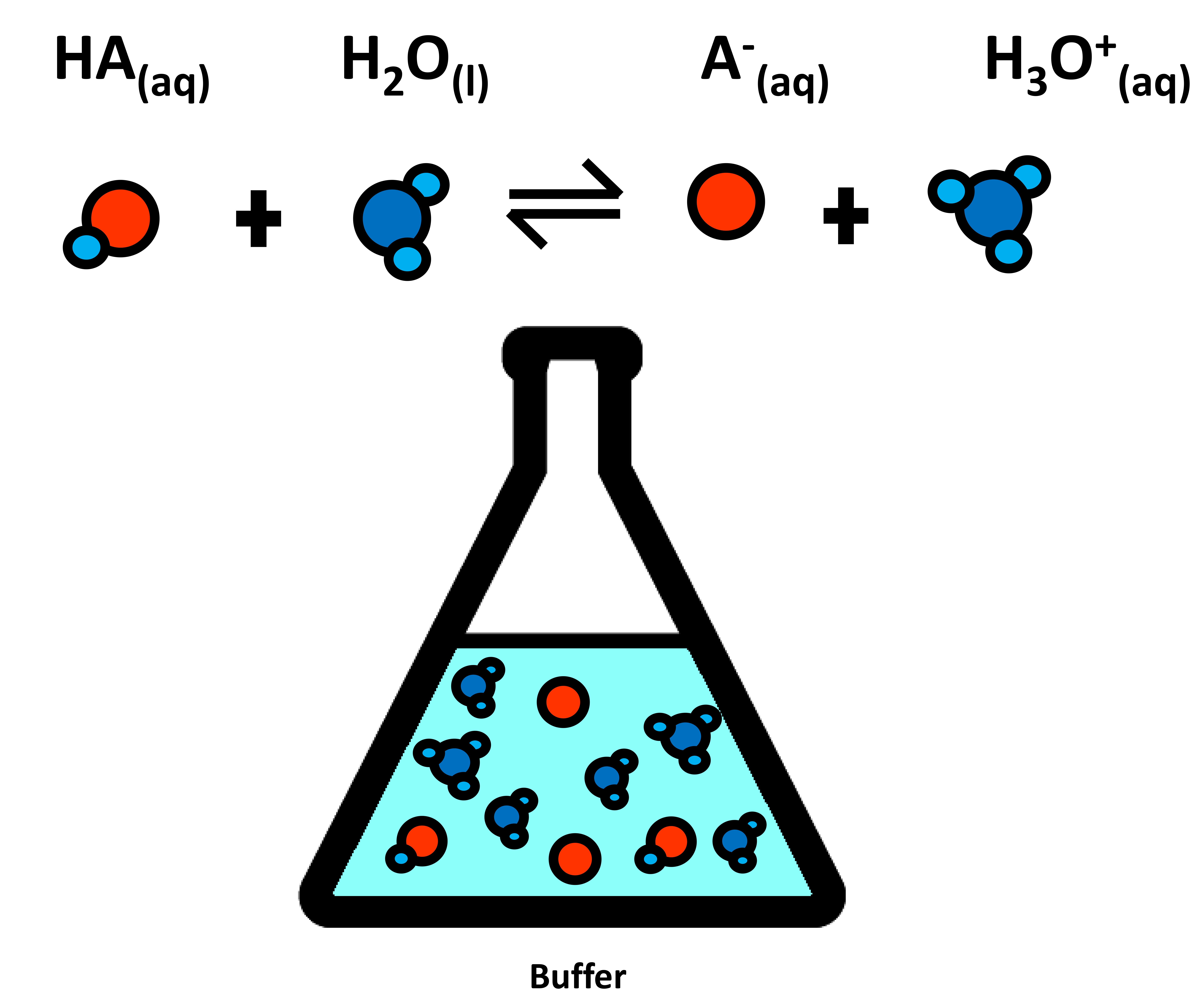
Which of the following represents a buffer system?. Which of the following represent a buffer system. We need a week asset with its conjugal base. So this is not a buffer system.
So this is not a buffer system. So in the first example we have just h three p 03 This is a weak acid but we do not have its conjugate base. Which of the following represent a buffer system.
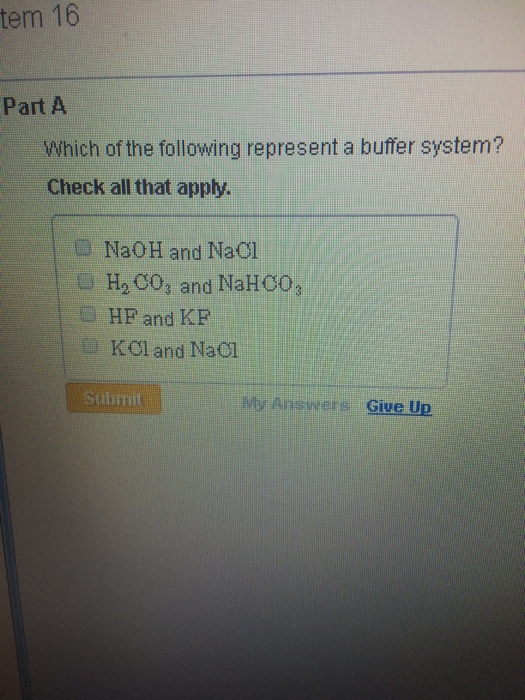
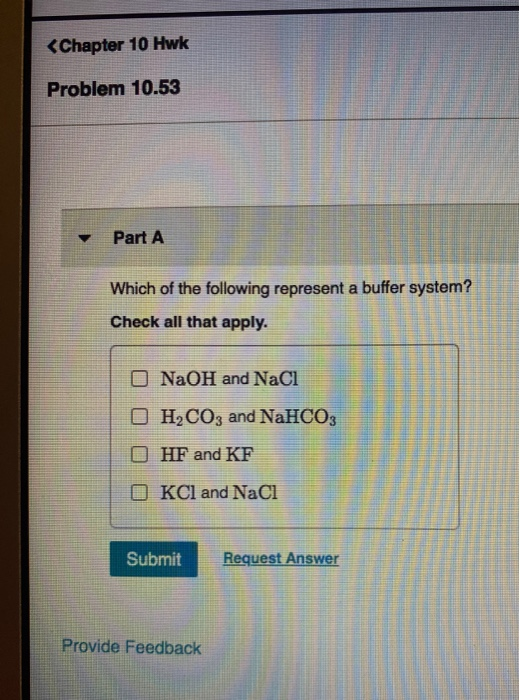
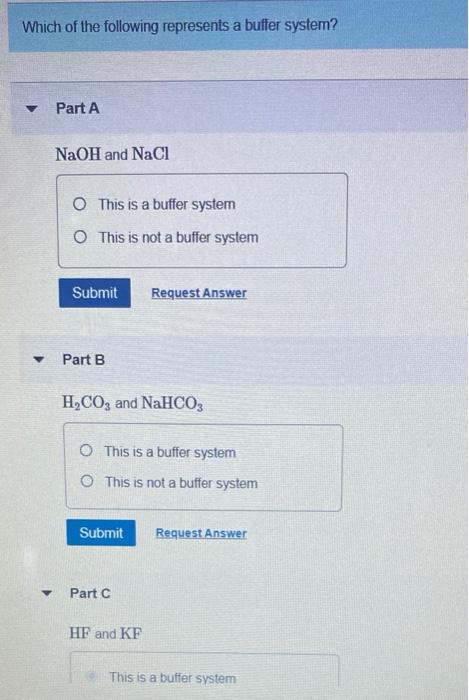
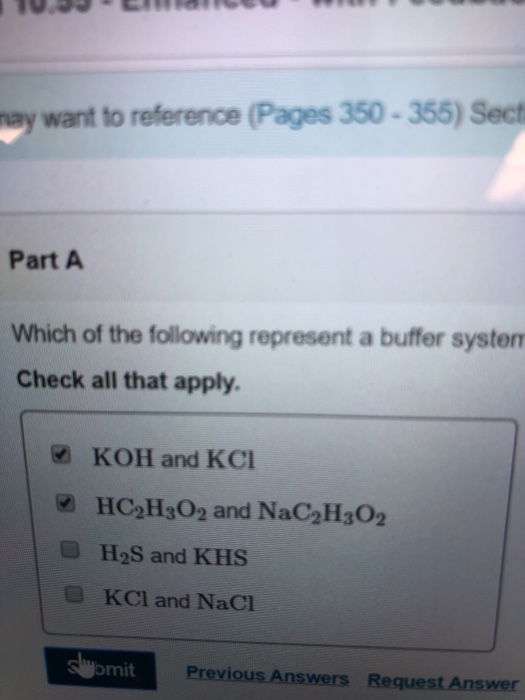
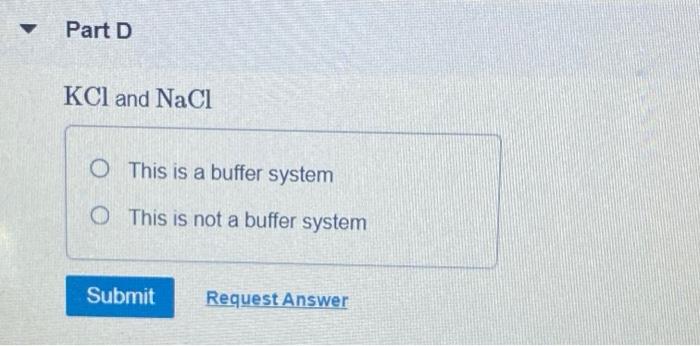
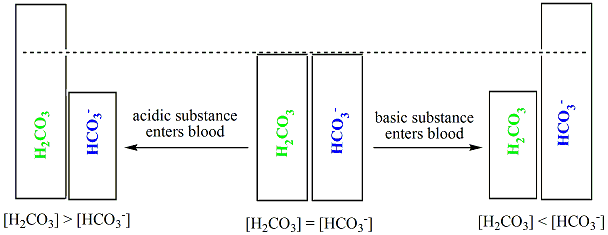
NaOH and NaCl HC2H3O2 and NaC2H3O2 H2S and KHS KCl and NaCl. Which of the following solutions represents a good buffer system. A CO2 H2O PictureH2CO3 PictureHCO3- H b CO2 H2O PictureHCO3- H PictureH2CO3 c H2CO3 PictureCO2 H2O PictureHCO3- H d H2CO3 PictureHCO3- H e CO2 H2O PictureHCO3- H.
We have a strong base. Check all that apply. Chapter 16 Exam-Type QuestionsMultiple Choice1.
But its so weak its weaker than water. Which of the following represent a buffer system. Which of the following represents a buffer system.
This is the best answer based on feedback and ratings. Im in salt sodium chloride so this is not a buffer solution. HF and NaF C.
Chemistry questions and answers. And the nitrate is also um a week base but weaker.
Science Chemistry Buffer solution.
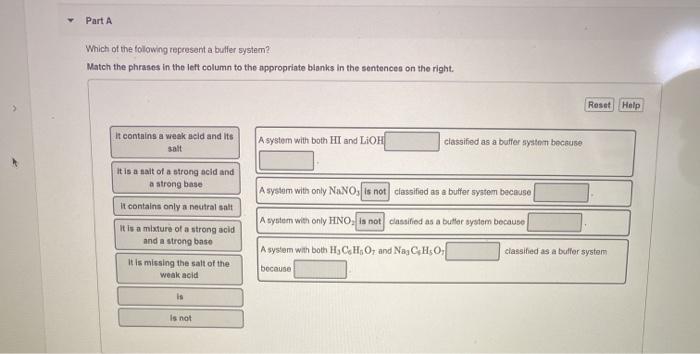
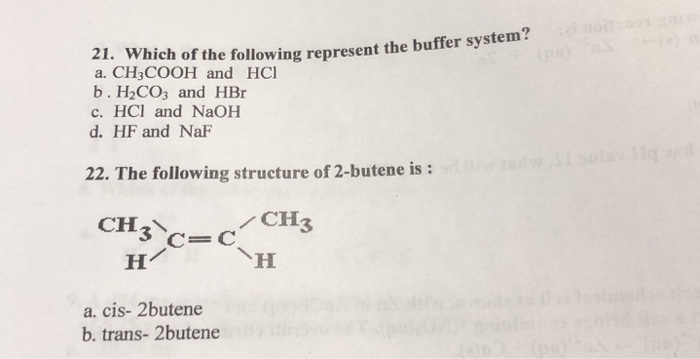
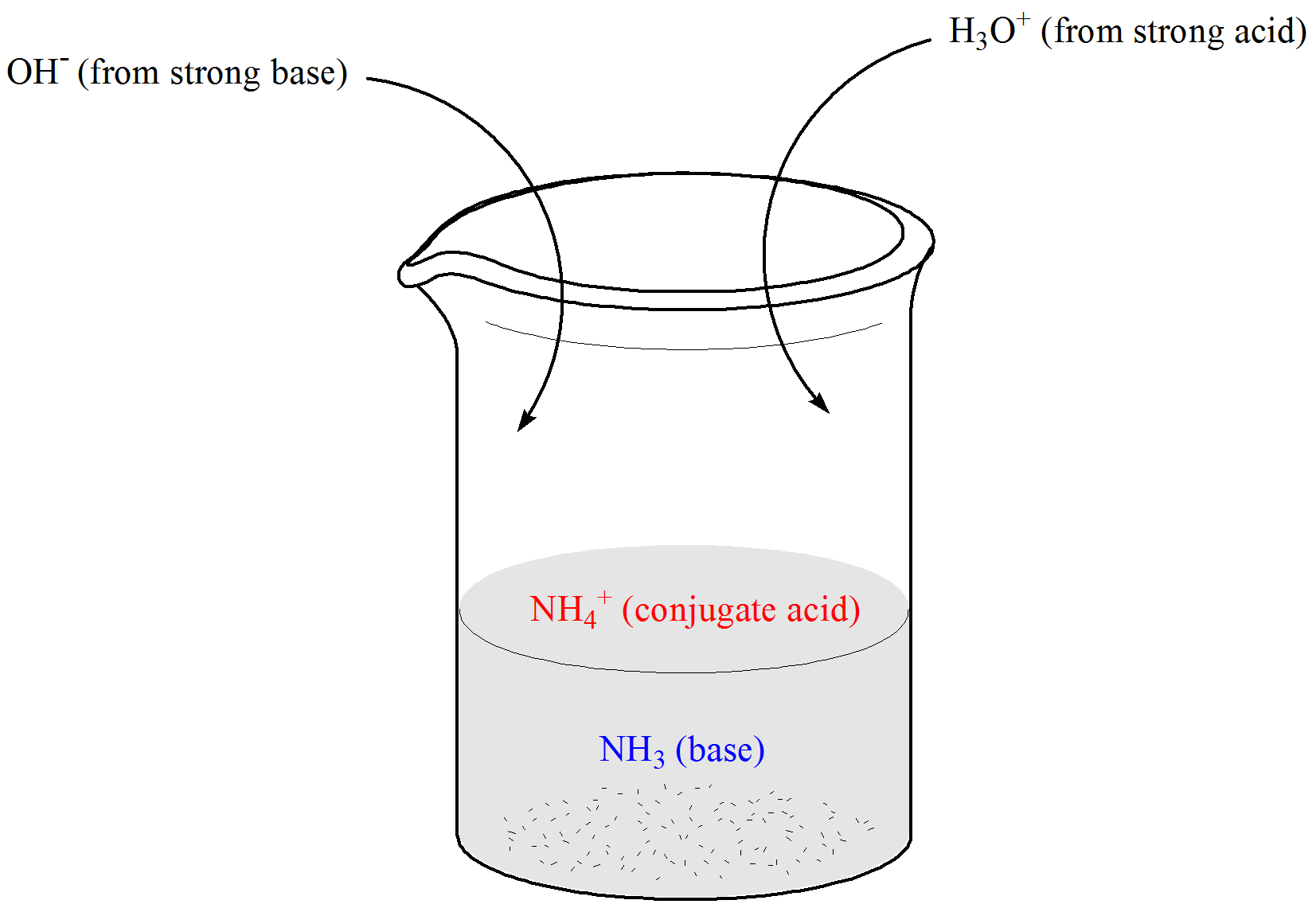
An isolated system is one in which objects inside the system are not influenced by any forces from outside the system. Which of the following represents a buffer system. And the nitrate is also um a week base but weaker. Chemistry questions and answers. Chemistry questions and answers. In order to have a buffer solution we need a weak acid with consequent base. Check all that apply. Explain aH3PO3 bNaNO3 cCH3COOH and NaCH3COO dHCl and NaOH. But its so weak its weaker than water.
So in the first example we have just h three p 03 This is a weak acid but we do not have its conjugate base. Check all that apply A. The 2nd 1 we have any and all three and a is not a um weak acid it is. H3PO4 NaNO3 CH3COOH and CH3COONa HCl and NaOH. Which of the following represents a buffer system. HC2H3O2 and C12H22O11 D. Science Chemistry Buffer solution.



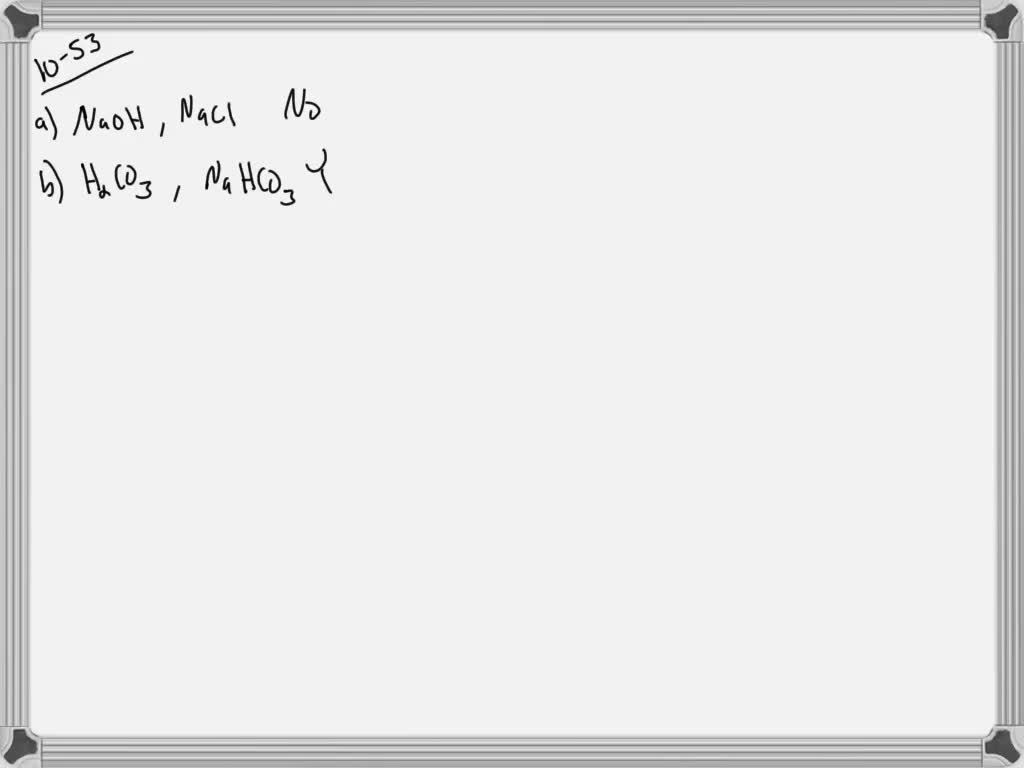























Post a Comment for "Which Of The Following Represents A Buffer System?"